Authors: Rajendra Kumar Yadav and Sunita Yadav
PhD. Scholar, Division of Soil Science & Agricultural chemistry, ICAR-IARI, New Delhi
Introduction:
Phosphorus (P) is an essential nutrient required by plants and animals in order to grow and maintain vital biological functions. Soils are mostly highly weathered and naturally low in P, which presents a significant challenge for agriculture because deficiencies of this element often limit growth and plant production. To overcome this challenge, applying a soluble form of phosphorus in fertiliser has been common practice in Australia since the 1920s, allowing farmers to cultivate economically viable crops and introduced pasture species. Application of P in fertiliser has been especially important in the nutrient deficient sandy soils of Western Australia for profitable wheat/grain production. Today, P is applied in excess of needs to some agricultural soils while, in other soils, the natural P sources in soils are effectively mined by crops.
Broader issues around P include concerns around the security of P supplies, the rising costs of agricultural production flowing from increasing global fertiliser prices and P leaching and runoff to the wider environment. This has encouraged development of more sustainable approaches to P management to deal with the on-farm and off-farm impacts of P use.
The behaviour of P in soils and the uptake and use of P by crops and pastures is important knowledge required by landowners and their advisers to help them sustainably manage nutrient requirements of crops. If P is not applied correctly, soil nutrient concentrations may exceed critical values (that needed to achieve near maximum yields) and increase the risk of nutrient leaching and runoff from the farm. This can result in the pollution of waterways, which impacts water quality and biodiversity through the associated degradation of ecosystem resources. This adversely affects the environment, and can result in a reduction of income to stakeholders through loss of ecosystem services.
Phosphorus in Soils:
There are complex processes that affect the movement of P in soils and its availability to plants. The different P pools and interactions in soil are summarised in Figure 1. The chemical reactions that move P between the different boxes or pools in Figure 1 influence the amount of P that is available to plants, such as the crops and pastures used in agriculture.
Only a small portion of the total P in soils (generally less than 1 per cent) is in the soil solution and can be taken up immediately by plants. This is replenished by the inorganic and other sources shown in Figure 1. It is generally considered that plants obtain their P largely from the soil solution as inorganic phosphate anions.
Phosphorus can exist in many different forms in soil, including inorganic and organic, that contribute in varying degrees to the plant-available pool (that which is readily available for plant uptake). There are three broad categories of P in soils. First, are the soluble phosphates that occur in soil solution and are readily available for plant uptake. Second, are those that become slowly available (labile), and third are those (non-labile) that become very slowly available (often over periods of many years) (Syers et al. 2008). The pools of organic and inorganic P shown in Figure 1 contain both labile and non-labile P.
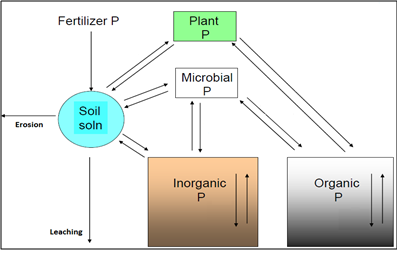
Figure 1 Phosphorus in soil (adapted from McLaughlin pers. comm. 2012)
Several factors can influence both the rate and amount of P taken up by plants. These factors include; the P concentrations in the soil solution, soil pH and the phosphorus buffering capacity of the soils (rate at which P in the soil solution is replenished, which is faster in soil with a high-buffering capacity). Phosphorus accumulated in soil as organic P is released to plants by several processes including mineralisation of organic matter and desorption from several fractions of inorganic P, but these processes are not yet well understood or quantified.
Soils adsorb P onto soil particles (what soil scientists call P-sorption), which reduces P availability to plants and thus adds to fertiliser requirement. The capacity of a soil to adsorb P is influenced by the surface area of soil and its composition, so that it is high in soils with fine particles and high clay content, and low in soils with coarse particles such as sands. In addition, inorganic P can undergo chemical reactions with other elements such aluminium and iron to form less soluble compounds that are less available to plants. In natural landscapes very small amounts of P are added to soils from very slow weathering of the parent rocks and in rainfall.
Various chemical tests can measure the amount of P in soils and estimate the amount potentially available to plants. Common tests that use different chemicals to extract the P from soil samples include the Olsen P and Colwell P (using bicarbonate extraction) but there are others such as Mehlich 3 and CaCl2 (Moody 2011). More recently developed tests include Diffusive Gradients in Thin-films (DGT) and sparingly soluble P reserves (BSES). In addition, there are techniques to refine these estimates of plant available P more reliably across wider environments using the phosphorus buffering index (PBI).
Phosphorus in Agriculture:
Significant amounts of P are removed from farms in the produce when sold. The highest rates of loss per area tend to be in vegetables, followed by milk, grains, fruit, meat livestock with very little in wool. If not replaced these removals lead to a rundown in P in soils. This happened in the soils in southern Australia cropped for grains leading to reduced yields from 1860 to 1910 and is currently occurring in the clay soils of north east Australia. Fertilisers, which contain concentrated forms of soluble P, provide a cost-effective means of compensating for these removals of P in produce.
However, not all the P applied in fertiliser is recovered by the pasture or crop plant. It can vary from about 5 to 40 per cent depending on the industry and other factors affecting plant growth. Some remains available for succeeding seasons, but the rest will be accumulate in soil and increase the level of available P and risk of loss through leaching (generally less than 5 per cent but sometimes over 40 per cent in sandy soils) and run-off in finer textured soils.
For farming systems to be sustainable and agronomically efficient, it is important to increase their P-balance efficiency. Ideally, this requires the amount of P removed in harvested product to be balanced by the equivalent input of a plant-available form of P. In practice, an allowance must be made for building soil P to critical values, for strong adsorption into the soil and any losses of P by leaching and run-off.
Many farms appear not to be following "best practice" fertiliser recommendations and are operating at soil fertility levels in excess of the level necessary for maximum production (Simpson et al. 2010). Soil P levels in 87 per cent of the cropping soils that undertook soil tests exceeded critical values (maximum production). While much of this excess can result in an accumulation of available P in the surface horizon of agricultural soils (Oehl et al. 2002) in some soils with low sorption capacity, such as in sandy soils, some can be lost through leaching. This application of P in fertilisers over 90 years has increased the amount of total and available P in agricultural soils (NLWRA 2001).
There is a net loss (or export) of soil P from some farming systems (Figure 4) where P is either not applied, or is supplied at rates and in forms that do not balance removal of P in plant products (Wong et al. 2012). Such agronomic practices are unsustainable, and depending on soil type, are usually associated (eventually) with declining yields over time. From an agronomic sense, soils which contain insufficient amounts of soluble-P not only produce economically unacceptable yields, but other inputs, particularly N and water, are used less effectively.
For instance, in the northern grains region, which is characterised by rotations and opportunistic sequences in winter and summer cereals, the application of P fertiliser to crops was often inadequate to maintain the soil P status and increasingly led to deficiencies of soil P. These P deficits tended to occur in lower (below 10 cm) soil layers because of zero tillage, shallow applications of fertilisers with seeds, an absence of P leaching and high reliance on stored soil moisture to grow crops. The financial risk for growers in this region from unpredictable rainfall and unreliable soil test methods for their subsoils may explain the continued low investments in fertiliser in this region (Wong et al. 2012; Simpson et al 2011).
This highlights the need to demonstrate to farmers and their advisers how soil fertility can be better managed using critical P values and appropriate fertiliser application strategies suitable for their farming system (Simpson et al. 2010).
Phosphorus in the Environment:
Improving the efficiency of P use in agriculture will also address some environmental concerns. Since around 75 per cent of P applied in Australian agriculture accumulates in the soil, some of this is then lost to the environment, with detrimental impacts on waterways. Phosphorus leaching and run-off into waterways leads to eutrophication (a change to the nutrient levels in the water) and results in a loss of aquatic biodiversity including the death of fish and plants. In many cases, these changes in the biological balance are seen first as algal blooms, which occur because of an increase in the concentration of bio-available P in the water (Syers et al. 2008).
There are three main pathways through which P leaves agricultural land and enters waterways and the off-farm environment. These are through erosion, surface water runoff and leaching (Hooper et al. 2000). Erosion can contribute significant amounts of P as particulate phosphates to waterways. Much of this P is not immediately available to the aquatic plants and probably has little impact on algal bloom formation in the short term. However, particulate inflows recharge the phosphate reserves present in the waterway sedimentary soils and these are released over time. This in turn stimulates plant growth and can contribute to sustaining or creating algal problems in the long term.
Surface water run-off has the potential to stimulate significant aquatic plant growth because the nutrients are already in solution and are immediately available to plants. Consequently, it has a more active role in the formation of algal blooms in the short term. However, a proportion of the phosphates in surface water runoff binds to sedimentary particles on entry, becoming unavailable to aquatic plants in the short term. As with eroded particulate phosphates, these phosphates are released over time.
Leaching can result in nutrients being washed down through the soil structure and into ground- and surface-water systems. Currently, there exists no accurate method for measuring the movement of P via this pathway at the broad acre level. Measurements on small plots suggest that leaching is important in acid sandy soils with low PBI. Improving P-balance efficiency will reduce input costs for farmers, and help improve water quality and aquatic habitats in estuaries, streams and wetlands.
Improving Phosphorus Management on farm:
It is apparent that managing P for both environmental and production benefits requires substantial knowledge and field testing. Phosphorus of agricultural industries and farming systems demonstrate that the use of P fertilisers is relatively inefficient and therefore careful fertiliser management strategies are needed. There is a therefore a need for simple effective methods allied with realistic goals.
Decisions about whether to apply fertiliser, how much and when, depend on the expected crop or pasture demand, and the ability of the soil to meet this under often variable climatic conditions. The decisions are also influenced by such factors as the availability of appropriate machinery, soil and crop type, rotation sequence, and the anticipated return on fertiliser investment.
One of the responses to these issues was development of a strategic framework to improve P management in the Australian grains industry (Wong et al., 2012). This framework identified issues impeding efficient P use and recommended short, medium and long term actions to address them.
The International Plant Nutrition Institute advocates “4 R” (expanded to “5 R” in Australia) of fertiliser management to support the farming system objectives for productivity, profitability, sustainability and the environment �" applying fertiliser as the:
• Right type (R1),
• Right amount (R2),
• Right time (R3) and
• Right place (R4) with
• Right agronomy (R5).
In the short term, large improvements in efficiency can be gained by working with farmers to demonstrate and implement the 5Rs. This requires communication and training, such as the FIFA FertCare program (or similar) to train advisers in better fertiliser management.
Options for improving the management of Phosphorus
Some options that have potential to advance these objectives are listed below.
Fertiliser modification to address R1: There is currently a limited choice of tested types of P fertilisers that could be used to improve efficiency in Australian agriculture. Several enhanced efficiency fertilisers are available and more are being developed. There are now fertilisers available that provide different rates at which P becomes available to match demand from annual or perennial crops and pastures depending on soil characteristics.
Using fluid fertilisers in calcareous soils can also be effective in improving P use efficiency. However, there are many products on the market that claim to increase the availability of existing recalcitrant P sources but are yet to be adequately tested for efficacy. Organic sources, including manures, are highly variable in their nutrient composition so that delivery of nutrients may be unbalanced for a crop’s needs. Suggested actions include:
• Evaluate and communicate the on-farm benefits of new types of fertilisers designed to improve on inefficiencies id different soils and farming systems, e.g. leaching P in sands, high P sorption in heavy clays
• Provide information on the P concentration of organic fertilisers, animal feeds, composts and animal manures to prevent under- or oversupply of P.
Phosphorus balances to address R2: The paddock to whole-farm P balance method provides easily understood outputs using relatively accessible information for farm management (Gourley et al 2012). The outputs should be combined with soil test values to provide a preliminary guide on management strategies to improve profits and environmental outcomes.
• The paddock and farm nutrient balance approach, such as used by Dairy Australia, could usefully be extended to all industry sectors to introduce the 5R strategy. It may be desirable to extend to an on-line nutrient mapping tool.
Soil testing to address R2 and R3: Predictions at the field level of the right rate of P fertiliser to apply require testing of soils to measure the amount of plant-available phosphorus in soils, and the buffering capacity of soils. Properly calibrated with field trials, these measures indicate a critical value of soil P required for pasture or crop production. In deficient soils, the soil tests can estimate the amount of P that should be added in fertiliser for immediate use by the crop for near maximum production, and to build up soil P levels. For soils with adequate P only maintenance amounts of P are required. As the concept of fertility maintenance is only a recent shift in emphasis, it will require demonstration and promotion to producers to improve their confidence in benefits to their profits and contain the potential for environmental pollution from over fertilising.
In some limited cases (perennials crops), plant tissue testing and other methods (such as the leaf colour chart) provide for in-crop monitoring for the right timing of P fertiliser to optimise either production or quality benefits. Suggested actions include:
• Extensive communication and training of consultants and farmers on the features and benefits of different soil and plant tests to better inform fertiliser management, for instance the where DGT tests may have a comparative predictive advantage.
• Work with farmers to demonstrate and promote the benefits of maintenance practice in paddocks that have reached or exceeded critical values of P. This will better determine appropriate rates of P for their soil, climate and farming system. In conjunction with programs such as the GRDC’s “Better Fertiliser Decision” and “More Profits from Crop Nutrition”, provide information on which soil test to use, how the data are interpreted to determine how much P to apply.
• Improve the availability and promote the use of critical values for P and calculators for advisers.
Fertiliser application to address R4: To apply fertiliser in the right place is a moving target that depends on pasture/crop grown, stage of development, soil fertility and management practices. For instance, with reduced-tillage systems less soil mixing means that P concentrates more in the top 10 cm of soil and may become deficient deeper in the soil. As this top section of a soil profile also dries fastest, it potentially limits the uptake and benefit from shallow applications of P fertilisers in dryland systems. Fertiliser placement in bands and deeper into the soil profile can provide greater recovery and production that may last for several years.
Suggested actions include:
• Providing a wider awareness of the possible consequences of changes in agricultural systems, e.g. reduced-tillage systems, for P nutrition of crops and pastures.
• Develop rules of thumb for identifying where P is deficient or sufficient for expected need within a paddock and that support the 5Rs.
Agronomic management to address R5: It is possible to increase the efficiency of P use with agronomic management that ameliorates non-P limitations to yields and that improves the uptake of soil P by increasing yields. Some strategies for improving agronomic management (Simpson et al 2012) include: overcoming other constraints to productivity (lack of other nutrients, acid soils, diseases etc; better targeting of fertiliser use to the critical P requirements of crop systems; careful use of organic matter to store and release P; reducing P accumulation in livestock “camps” through grazing pressure; selecting crop and pasture plants more tolerant of low P concentrations in soil and plants that produce well at lower critical P levels. with higher P use efficiencies; or the adoption of low-P farming systems. Suggested actions include:
• Conduct P footprint analyses of major agricultural industries and regions to refine actions for combining agronomic with environmental outcomes.
• Develop packages of information suitable for farm managers and their advisers that show how current knowledge of P management fits within best practice nutrient management on farms. The packages, customised for different agricultural industries, should aim to improve both farm productivity and reduce losses to the wider environment and include difficult soils (e.g. sodic, acidic).
About Author / Additional Info:
Ph.D Scholar, Division of Soil Science and Agricultural Chemistry, IARI New Delhi ,India