Author: Pawan Singh Gurjar
Introduction
Fruits are important sources of digestible and indigestible minerals, carbohydrates and certain vitamins, particularly vitamins A and C. The moisture in most of the fruits above 75% and fruits are prone to spoilage by molds and yeasts (Janisiewicz et al., 1999). Fruits and vegetables are produced during peak seasons but due to lack of preservation and storage facilities, the market become overstocked during such periods and get rotten prior to reach the final consumer.
Osmotic dehydration has been successfully used to reduce water activity of fruits and vegetables to about 0.9, keeping much of original quality. Osmotic dehydration is now considered a valuable tool in minimal processing of foods. It can be applied either as an autonomous process or as a processing step in alternative processing schemes leading to a variety of end products (Lazarides et al., 1995). During osmotic dehydration, a product continuously immersed in the osmotic solution, making the process oxygen free. Therefore, no need to use sulfur dioxide and/or blanching for protection against oxidative and enzymatic discoloration. Also, the process takes place under mild heat treatment (<50◦C) which further improve color and flavor retention, resulting in products with superior sensory characteristics. Osmotic dehydration is, therefore, one of the effective ways to reduce overall energy requirements in dehydration and dehydro-freezing processes. Osmotic dehydration has been combined with conventional drying methods such as hot air drying to produce shelf-stable fruit products. Osmotic dehydration process has been applied to many fruits and vegetables viz. apple, apricot, banana, carrot cherry, citrus fruits, grapes, guava, papaya, mango, potato, etc.
Principles of osmotic dehydration
The principle underlying osmotic dehydration is that water diffuses from dilute solution (Hypotonic solution) to concentrated solution (Hypertonic solution) through a semi-permeable membrane till equilibrium is established. The driving force for water removal is the concentration gradient between the solution and the intracellular fluid. If the membrane is perfectly semi-permeable, solute is unable to diffuse through the membrane into the cells. However, it is difficult to obtain a perfect semi-permeable membrane in food systems due to their complex internal structure and there is always some solid diffusion into the food which means that osmotic dehydration is actually a combination of simultaneous water and solute diffusion process (Fig. 1).
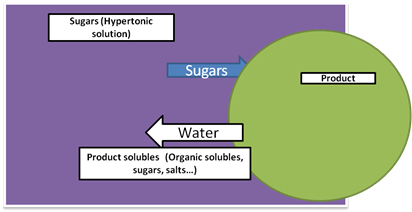
Figure 1. Mass transport in osmotic dehydration
Factors affecting osmotic dehydration process
Type of osmotic agent: The most commonly used osmotic agents are sucrose for fruits. Other osmotic agents include glucose, fructose, lactose, dextrose, maltose, polysaccharides, malt dextrin, corn starch syrup, etc. The osmotic agent used must be harmless and have a good taste. Lower molecular mass saccharides (glucose, fructose, sorbital, etc.) favour the sugar uptake because of the high velocity of penetration of the molecule so that solid enrichment instead of dehydration is the main effect of the process.
Concentration of osmotic solution: The kind of sugar and its concentration as osmotic substance strongly affect the kinetics of water removal, the solid gain and the equilibrium water content. By increasing the molar mass of solute, weight loss and dehydration aspects of the process are favoured and both equilibrium and drying rate increases with the increase of osmotic syrup concentration.
Temperature of osmotic solution: The rate of osmosis is markedly affected by temperature. This is the most important parameter affecting the kinetics. The water loss increases with the increase of temperature, whereas solid gain is less affected by temperature. The rate of osmosis increases the mass exchange and diffusion coefficient but above 50◦C, enzymatic browning and flavor deterioration takes place (Videv et al., 1990).
Agitation of osmotic solution: Osmotic dehydration increases when the syrup is agitated or circulated around the sample. This is due to reduced mass transfer resistance at the surface. The agitation-induced decrease in the rate of solids gain for longer osmosis periods could be an indirect effect of higher water loss (due to agitation) altering the solute concentration gradient inside the food particle.
Geometry of the sample: Osmotic concentration behavior will depend on the geometry of sample piece. This is due to the variation of surface area per unit volume or mass and diffusion length of the component involved in mass transport.
Osmotic solution and food mass ratio: Both solid gain and water loss increased with the increase of syrup and food mass ratio up to a certain level and then falls off. To avoid significant dilution of the medium and subsequent decrease of the (osmotic) driving force during the process a large ratio (at least 30:1) should be maintained.
Physical and chemical properties of food materials: The chemical composition (Protein, carbohydrate, fat, salt, etc.) and physical structure (porosity, arrangement of the cell, fiber orientation, and skin) may also affect the osmotic kinetics in food.
Time of Treatment: The loss in water content and gain in soluble solids content is a function of time. In general, as the time of treatment increases, the weight loss increased but the rate at which this occur decreases. Maximum water loss (50% reduction) takes place within the first two to three hours depending upon the type of fruits.
Advantages of osmotic dehydration:
- Quality improvement in terms of colour, flavour, or aroma and texture.
- Energy efficient as compared to other dehydration techniques namely air, vacuum, and tray drying as it can be conducted at low or ambient temperatures.
- Reduction in packaging and distribution costs.
- Reduces enzymatic browning. More product stability during storage due to low water activity by solute gain and water loss.
- Flavour retention is also more when sugar or sugar syrup is used as an osmotic agent.
- There is minimum loss of colour, flavour, and nutrient as it is low temperature process.
Conclusion
The osmotic dehydration method is very useful as it results in high quality product by retaining the colour, flavour and other volatiles. The overall energy requirement in drying process is reduced substantially. Fruits like pineapple, apple, strawberry, papaya, mango, banana, melon, apricot, peach and pears can be osmotically dehydrated successfully for removing initial 30-40 % moisture. The recent developments in the osmotic dehydration has reduced the time of osmosis and increased the moisture loss with controlled solid gain. The mathematical models of osmotic dehydration of most of the fruits can be used for understanding the osmotic dehydration process of fruits properly. It can lower the operating costs of the fruits drying process.
References:
- Janisiewicz, W. J., W.S. Conway and B. Leverentz. (1999). Biological control of postharvest decays of apple can prevent growth of Escherichia coli O157:H7 in apple wounds. Journal of Food Protection. 62: 1372-1375.
- Pokharkar, S. M. (2001). Kinetic model for osmotic dehydration of green peas prior to air-drying.Journal of Food Science and Technology, 38(6): 557-560.
- Videv, K., Taucher, S., Sharma, R. C. and Joshi, V. K. (1990). Effect of sugar syrup concentration and temperature on the rate of osmotic dehydration of apples.Journal Food Science and Technology, 25: 307–308.
About Author / Additional Info:
I am working as Scientist in Division of Post Harvest Management at ICAR-CISH, Lucknow.