Author -Sandhya Sanand, Kishor U Tribhuvan, Anshika Tyagi and Kishor Gaikwad
ICAR- National Research Centre on Plant Biotechnology, New Delhi - 110012
Basic principal: Mass spectrometer is an analytical tool which fragment the sample into multiple ions and then separate them according to their m/z ratio, on the basis of relative abundance of ions, molecule under investigation will identified and quantified. It is popularly used for identification of biomolecules particularly protein in a complex mixture. Instrumentation: Mass Spectrometers consist of three majors component
- 1. Ion sources
- 2. Analyzer
- 3. Detector system

Fig1. Component of mass spectrometer Ion Source:
Ion source is used for production gaseous ions from sample of investigation. As samples are introduced in to ionization sources via inlet system, sample molecules are ionized in to ions in the presence of high voltage. The fragmentation of molecules occurs in presence of high voltage because at high voltage energy of electron increases which leads to collision of molecules and electrons. Ionization may be electrospray, Nano spray, chemical based, matrix assisted laser desorption, fast atom bombardment, thermal ionization, filed ionization, field desorption etc. The working principles of some of ion sources are as fallow
Electrospray ionization (ESI): In Electrospray ionization sample under investigation is mixed with volatile solvent and pass through a small capillary, at the nozzle of capillary high voltage is applied which evaporate solvent molecule and provide maximum charge to the sample molecules for production of ions. The capillary made up of stainless steel with diameter of 0.02 to 0.01 mm and length 60 mm with flow rate 1–20 μL/min. After injecting the sample through inlet, ionization of sample is started by applying 2-6 Kv voltage near the tip of the capillary and heated at 100–300°C, which causes the complete desolvation of the ions. The size of primary charged droplets of 1-2 μm in diameter which further evaporate to form ions. The use of drying gas and the heated capillary can affect the system’s robustness and also decrease the degree of cluster ion formation.
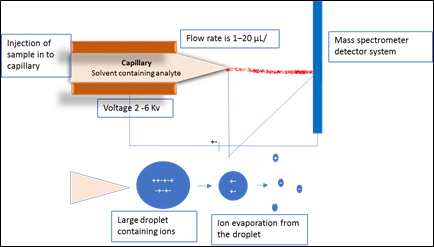
Fig2. Schematic representation of ionization via electro spray method
Nanospray ionization: This is a modified version of electrospray in which the spray voltage of 0.7–1.1 kV is normally applied through an electrically conducting coating (usually a sputtered gold film) on the outer surface of the spray capillary. The flow rate is high as compared ESI (20–50 nL/min). Stainless steel capillary is replaced by fine tip (1-4 μm diameter) borosilicate glass capillary. The size of primary charged droplet via nanospray is in the nanorange (≤ 200 nm). Less amount of sample required for nanospray ionization as compared to electrospray ionization. Matrix Assisted Laser Desorption Ionization (MALDI): This is quite complex method of ionization as compared to electrospray because it depends upon light absorbing matrix. Sample under investigation is mixed with light absorbing matrix like 3,5-dimethoxy-4-hydroxycinnamic acid (sinapinic acid), α-cyano-4-hydroxycinnamic acid (alpha-cyano or alpha-matrix), 2,5-dihydroxybenzoic acid (DHB). The mixture of matrix and sample is bombarded with laser light, light energy is absorbed by matrix and pass to sample molecule. Due to gain of energy production of protonated ions is started. These protonated ions are accelerated and separated on m/z ratio.
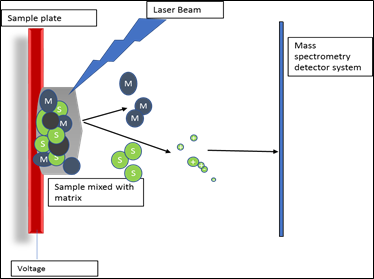
Fig3. Schematic representation of ionization via MALDI method Analyzer
After formation of ions from the sample, analyser separate them according to their mass-to-charge (m/z) ratio. Example of analyser includes Time of Flight (TOF), Quadrupole, Quadrupole Time of Flight (Q-TOF), Q-TOF-TOF, magnetic, ion trapped etc.
Time of Flight (TOF): analyse ions on the basis of time taken by ions after accelerated by electric field to travel from flight tube to detector tube. It is calculated by the formula: TOF= k/√ m/z
Quadrupole: It consist of four hyperbolic rods through which gaseous phase ions has to achieve a stable distance. Application of alternative electric field help to stabilize and destabilize passing ions. Ion travels to space between the rods, specific voltage is applied through rod helps to pass a ion with specific m/z value and reach the detector.
Detector System It is final element of the mass spectrometer which record either charged induced or the current produced when ion hit a detector surface. An ideal detector system characteristics is having high amplification, fast response, low noise background, stable and cost effective. Detector may be photomultiplier, photo multitube, electron multipliers, faraday cups etc. All detector system is not compatible with all the instrument. The compatibility of analyser and detector system varies from instrument to instrument. The choice of detection is based on need of sensitivity and speed.
Applications of Mass Spectrometry:
- It is used to study post translation modification like structure of protein, protein folding and protein interaction analysis.
- It is used to identify mass of different isotope.
- It is a very important component of proteomics, widely used for identification of protein fragment
- It is used to analyse biomolecule like lipid, protein, peptide, glycolipids, oligonucleotide etc.
- It is also used in forensic toxicology
- It is used in clinical and pharmaceutical industry to test food and beverages for contamination, adulteration and also product consistency.
- It is used for analysis of soil sample contaminants.
- It also used analysis of trace gases.
Conclusion: Sample preparation for MS analysis is very cautious. Because of its high sensitivity and accuracy, they are being used in analytical laboratories for studying physical, chemical and biological properties of a given sample.
References:
Singhal N, Kumar M, Kanaujia P K, Virdi J S(2015) MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. 6: 791
Banerjee S and Mazumdar S, Electrospray Ionization Mass Spectrometry (2012) A Technique to Access the Information beyond the Molecular Weight of the Analyte. International Journal of Analytical Chemistry. Article ID 282574
http://www.astbury.leeds.ac.uk/facil/MStut/mstutorial.htm
About Author / Additional Info:
I am currently working as a Scientist at ICAR-National Research Center On Plant Biotechnology, New Delhi