Authors: PARIMAL RAMESH UDGAVE
ICP-OES (Inductively coupled plasma optical emission spectrometry) is also known as Inductively coupled plasma atomic emission spectroscopy (ICP-AES) which is used to detect the trace metals in particularly water dissolved samples. It uses inductively coupled plasma which excites the ions from the sample element. ICP-OES has main two parts: ICP and Optical spectrometer. Inductively coupled plasma is produced from the torch which made up from 3 concentric quartz glass tubes (Hieftje et al. 1982). Argon is burned to create the plasma.
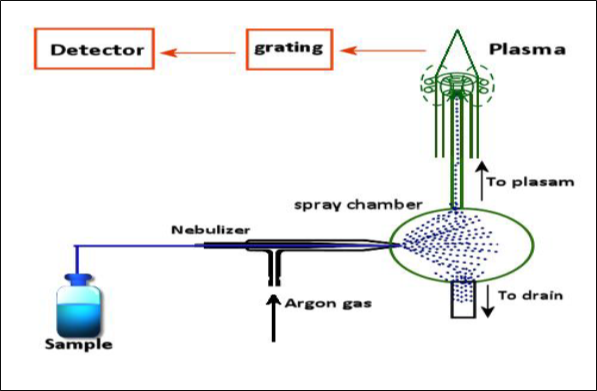
Figure 1. Working of the ICP-OES from the sample introduction to detector (Mao et al. 1989).
The working of the instrument is explained briefly here:
A Peristaltic pump transports the aqueous or organic sample to the analytical nebulizer. The function of this analytical chamber is to convert the sample into mist and to introduce into the plasma flame. This results into the collision of the sample with the electrons and it is broken down into charged ions (Mao et al. 1989). This breakdown induces radiation in particular wavelengths according to the sample element. In next step, the radiated light is separated into different wavelengths in optical chamber. This light is measured by a photomultiplier tube which consists of semiconductor photodetectors (Charged coupled devices: CCDs). These detector arrays can measure the intensity of all wavelengths which allows analysis of every element and quick analysis. This intensity is matched and compared with the known concentration of element which is included in the calibration curve of the instrument. The accuracy of the instrument is dependent on the calibration curve because the intensity from the range of known concentrations will be matched to the element in the analyte.
For the ICP-OES analysis, the sample preparation can be done in different ways. For instance, dry ash digestion, wet oxidation digestion, and microwave system digestion. However, microwave system digestion is more effective than other methods. The advantages are simple, fast and safe to use the method. A variety of acid can be used for the microwave system digestion, however, nitric acid with hydrogen peroxide digestion is commonly used for plant tissue analysis.
Sample preparation:
Grown plants were taken from sand hydroponic pots. Seedlings were carefully removed and washed with deionized water and dried on tissue paper towel. The plant biomass was weighed by using electronic balance and noted for observations. These plants were cut separately in three parts roots, shoot, and leaves. Approximately 0.30 g of each root, shoot, and leaves of the seedlings were taken and ground with mortar and pestle and added to separate microwave digestion vessels. These microwave digestion vessels were cleaned by using this method: Microwave digestion vessels were rinsed firstly with deionized water and then kept for 18 hours in 10 % nitric acid bath and subsequently rinsed with deionized water and dried by using the oven at 600c. Nitric oxide (70%) and hydrogen peroxide (30%) were used for the digesting plant samples. In each microwave digestion vessels, 1.5 ml of nitric acid and hydrogen peroxide was added and gently shaken for mixing with ground plant sample and kept for 10 minutes in a fume hood. In next step, these vessels were kept in microwave digestion vessels and ran by using plant material mode. Each run took 20 minutes for digestion and 20-30 minutes for cooling. After digestion, these vessels kept in a fume hood for cooling (10 minutes). In a further step, the vessels containing digested plant material sample were transferred to 50 ml volumetric flasks and diluted with deionized water and final volume made 50 ml per sample and stored in it.
2.5.2 Calibration strategy:
The silver standard for ICP analysis was obtained from Sigma- Aldrich UK Ltd. Different concentrations of 100 ml were prepared for calibration of ICP-OES. These concentrations were 0.1 ,0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 μg/ml.
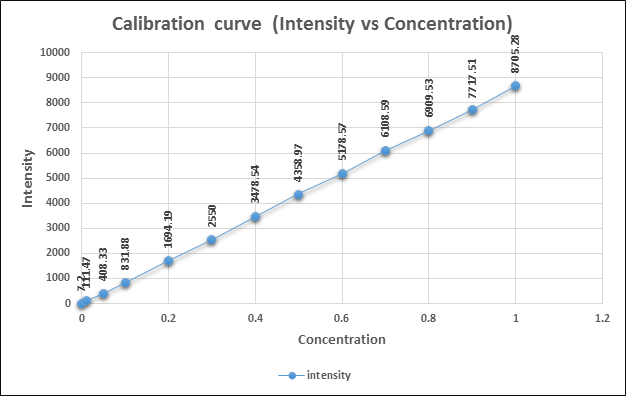
Figure No. 2 Calibration curve for ICP-OES (Intensity vs Concentration
Limit of detection of ICP-OES:
The detection limit of ICP-OES is calculated using the following formula:
LOD= k x BEC x RSD0
Where, LOD= limits of detection
k = 3 for the normal 3-sigma values
BEC = Background equivalent concentration
RSD0= relative standard deviation of the blank
To calculate the limit of detection of ICP-OES instrument, the value of k and BEC was obtained from calibration curve report using two points i.e. 0 μg/ml and 10 μg/ml and for the RSD0 value the blank was ran for 10 times and the average value of 10 RSD0 was taken as RSD0.
The obtained values are
K=0.008,
RSD0=0.56,
BEC= 3.54 μg/ml.
LOD= 0.008x3.54x0.56 = 0.01 μg/ml
Results:
Showing silver ion content in Barley grown in sand hydroponics
| Treatment/ Samples | Root (μg/ml) | Shoot (μg/ml) | Leaves (μg/ml) |
| Control | 0 | 0 | 0 |
| AgNP20 | 1.66 | 1.66 | 1.66 |
| AgNP40 | 1.66 | 1.66 | 0 |
| AgNO3 | 60 | 1.66 | 1.66 |
| AgClo4 | 83.33 | 5 | 1.66 |
Barley seeds treated with silver nanoparticle 20 nm, 40 nm, and silver nitrate indicated a similar silver concentration in the shoot (1.66 μg/ml). The highest silver content (5μg/ml) was achieved in seeds treated with silver perchlorate treatment and the lowest was 0 in shoots of control seeds.
In the leaves of barley seeds grown in sand hydroponics media, the control and silver nanoparticle 40 nm treatment indicated the absence of silver ions. However, a silver nanoparticle with 20 nm, silver nitrate and silver perchlorate showed 1.66 μg/ml silver concentration.
References:
1. Hieftje, G. (1982). Design and Construction of a Low-Flow, Low-Power Torch for Inductively Coupled Plasma Spectrometry. Applied Spectroscopy. 36 (6), 627-631.
2. Mao, H., Hieftje, G. (1989). Simultaneous measurement of spatially resolved electron temperatures, electron number densities and gas temperatures by laser light scattering from the ICP. Spectrochimica Acta Part B: Atomic Spectroscopy, 44 (8),739-749.
About Author / Additional Info:
I am working as Asst. professor. My scientific interests include Nano-biotechnology, Soil toxicology, Eco-toxicology and Environmental science.