Author: Namita Das Saha
Centre for Environment Science and Climate Resilient Agriculture (CESCRA)
Indian Agricultural Research Institute (IARI), Pusa, New Delhi-110012, India
Introduction:
Human being are the most intelligent creature on this earth, employ social networking and communication in a well-organized way. Although it was a common perception that microbes behave like a solitary organism but, in last two decades it has become clear that single-celled microbes can even communicatewith each other and respond as well behave collectively to achanging environment. They behave like a well-organized social organism in a cooperative manner. Such a cell-to-cell communication mechanismis known as quorum sensing (QS) and in manycases, plays essential roles in synchronizing certain set of gene expressionand thus functional coordination among bacterial communities. This is like chit-chat between organisms of same species and as well other species. The bacteria release, detect and respond to accumulation of signal molecules in a cell density-dependent manner,thereby regulating the expression of a set of targetgenes. For several purposes, they communicate in such a way viz., biofilm formation, conjugation, motility, sporulation, pathogenesis, antibiosis etc.
Types of signal molecules:
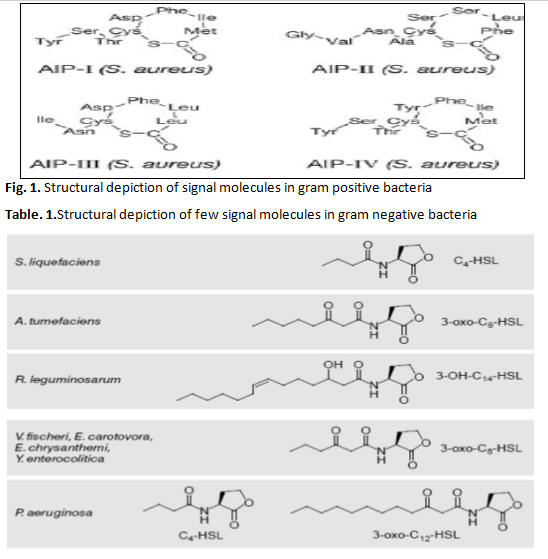
In general two types of signal molecules are there. Short chain peptides are specific type of signal molecule for QS in case of gram positive bacteria (Figure. 1) and acyl homoserine lactone (fatty acids derivatives) in case of gram negative bacteria. However, several derivatives of these two basic types of bacterial cell-to-cell communicationsignals have been identified in the last two decades, such as N-acylhomoserine lactone (N-AHL), cyclic thiolactone(AIP), hydroxyl-palmitic acid methyl ester (PAME), furanosylborate (AI- 2) and methyl dodecenoic acid (DSF), most of which are involved in theregulation of bacterial virulence.In case of gram negative bacteria, all types of signal molecules possess a resemblance in structure among themselves. There is similarity in the lactone homoserine moiety and only difference is in the side chain length and different functional groups substituted to the side chains (Table. 1). The QS phenomenonis not only limited to the prokaryotic kingdom;single-celled eukaryotic fungal pathogens can also use QSsignals to coordinate biological functions. It has recentlybeen reported that the fungal pathogen Candida albicansproduces farnesoic acid (FA) to regulate the yeast-tomyceliumtransition that is important for the fungal virulence.
Discovery of Quorum sensing:
The concept of quorumsensing (QS)was first elucidated inthe luminescent marine bacterium Vibrio fischeri.It wasdiscovered that these organisms express genes controllinglight emission only when associatedwith their fish or squid symbiotic hosts. When freeliving in the ocean (i.e. at low cell density), luciferase is notexpressed. When grown in culture in the laboratory, luciferaseexpression was rapidly induced in a coordinatedmanner at the late log phase of cell growth. Attempts touncover the mechanism of this gene regulation involved experiments in which conditioned, cell-free culture mediafrom bacteria growing at high cell density was added to alow-density culture (Nealson, 1970).Cells growing at low densityexpressed luciferase in the presence of the conditionedmedia.Thus itwas discovered that a factor secreted by cellsaccumulates as cell density increases, and this factorinduces expression of the luciferase-encoding genes. Thiswas the first demonstration of QS, or cell population density dependent control of gene expression.
Although initially discovered in marine symbionts, QS systems are also employed by pathogenic bacteria to altergene expression coordinately in response to associationwith a eukaryotic host organism. Many human pathogens, including Pseudomonas aeruginosa, Burkholderiacepacia, Salmonella typhimurium and Yersinia enterocolitica, regulateexpression of virulence factors required for pathogenicityvia QS. P. aeruginosa is one of the most studied QSorganisms because of its role as an emerging opportunistichuman pathogen.
Specific mechanism of bioluminescence in V. fischeri :
In case of Vibrio fischeriLuxI/LuxR quorum sensing regulon system regulates the bioluminescence. There are six luciferase structural genes ( luxCDABEG) and two regulatory genes luxIand luxRrequired for quorum sensing control light emission in Vibrio fischeri. The genes are arranged but divergently transcribed units. The LuxI protein is responsible for synthesis HSL auto inducer. At low population density, luxI and luxR are transcribed at a low level and there is insufficient accumulation of quoromone signal to elicit the LuxR dependent transcription of lux operon for visible luminescence. As the cell population density increases, concentration of autoinducer also increases both in intra and extracellularly. At a critical autonducer concentration the LuxR protein bind to autoinducer and this complex binds to the lux promoter to activate the transcription of operon and ultimately there is exponential increase in the light production via increase in the transcription from lux operon (Fig. 2).
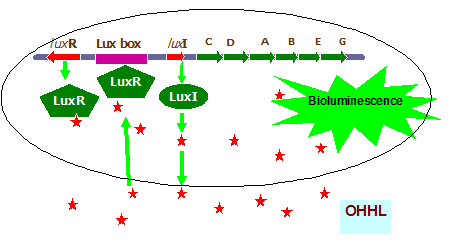
Fig. 2. Schematic diagram of quorum sensing controlled bioluminescence production in Vibrio fischeri
Different purposes for which Quorum sensing is employed by bacteria:
For different purposes bacteria do quorum sensing among themselves viz., biofilm formation, pathogenesis, symbiosis,production of secondary metabolites, dispersal and dissemination, conjugation, competence, motility, sporulation.
Quorum quenching:
Breaking of the quorum sensing circuit is called Quorum Quenching (QQ). This QQ can be achieved at three different stages. First, targeting to precursor for signal production. Second, targeting to the dissemination of the signals and third, targeting to the reception.
The first quorum quenching enzyme (AHL lactonase) encoded bythe aiiAgene was identified from a soil bacterial isolatebelonging to a Gram-positive Bacillus species-2401B, which was later characterized as an AHL-lactonase(Dong et al., 2001).
Diversity of microbes producing QQ enzymes:
Subsequently, a range of other bacterial isolates and strains that produce AHL degradation enzymes have beenidentified from soil, plant and biofilm samples as well asfrom laboratory bacterial culture collections. Quorum quenching enzyme activity has so far been demonstratedand documented in at least 10 bacterial species,including 4 Bacillus species, Agrobacterium tumefaciens,Arthrobactersp., Klebsiellapneumoniae, P. aeruginosa,Rastoniasp. and V. paradoxus.The correspondinggenes encoding the AHL-degradation enzymes havebeen cloned and characterized in most cases. Interestingly,these organisms taxonomically belong to three phyla ofthe BacteriaKingdomi.e., Actinobacteria(Arthrobactersp.), Firmicutes(Bacillus species) and Proteobacteria (A. tumefaciens, K. pneumoniae, P. aeruginosa, Ralstoniasp.. and V. paradoxus).Such a diverse distribution suggests that thegenes encoding AHL-degradation enzymes might bewidely conserved among many prokaryotic organisms.
Acyl Homoserine Lactone (AHL) degradation mechanism through AHL lactonases:
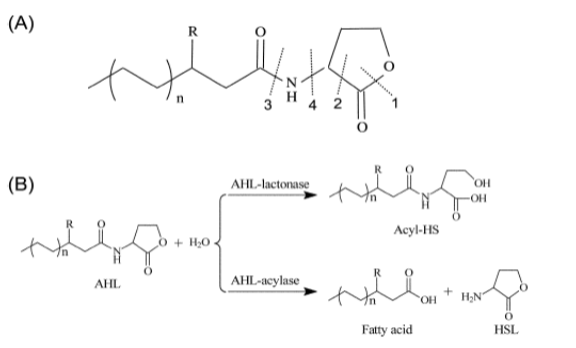
Fig. 3. The general structure of AHL signals and enzymatic degradation products. (A) The AHL structure and its possible enzyme cleavagesites; (B) the correspondingdegradation mechanisms of AHL-lactonase and AHL-acylase. The enzymes in 1 and 3 positions only yet identified and the structural study depicts that in 2 and 4 position there is chances of action of other two enzymes which are yet to be characterized.
Conclusion:
Although the study of quorum sensing is only at its beginning, we are now in a position to gain fundamental insight into how bacteria build community networks. This system can be exploited for better management of diseases spread by different pathogenic bacteria.
References :
1. Dong, Y.-H., L.-H. Wang, J.-L. Xu, H.-B. Zhang, X.-F. Zhang, and L.-H. Zhang. 2001. Quenching quorum sensing-dependent bacterial infection by an N-acyl homoserinelactonase. Nature 411:813â€"817
2. Nealson K.H, Platt T, Hastings W.1970. Cellular control of the synthesis and activity of the bacterial biolumionescent system. Journal of Bacteriology 104, 313â€"322.
About Author / Additional Info:
I am working as scientist at CESCRA, ICAR-Indian Agricultural Research Institute, New Delhi